Imagine that every cell in your body has an internal clock silently counting down time. This clock does indeed exist, and it bears a name: telomeres. These DNA sequences that cap the ends of your chromosomes shorten with every cell division, directly influencing your aging process and overall health. Discover in this article how these microscopic structures determine your biological age, why they represent a key factor in longevity, and above all, what concrete actions you can take to protect them and optimize your health capital in the long run.
Summary
- Telomeres: understanding your cellular biological clock
- The link between telomeres and the aging process
- Factors that accelerate telomere shortening
- How to protect your telomeres?
- Telomerase: the longevity enzyme
Telomeres: understanding your cellular biological clock
Telomeres are repetitive DNA sequences located at the ends of your chromosomes. The term comes from the Greek “telos” (end) and “meros” (part), literally referring to the final part of the DNA. Their main function is to preserve the integrity of your genetic heritage by protecting chromosomes during cell replication.
Specifically, telomeres act like the plastic tips on your shoelaces: they prevent fraying and deterioration of more important structures. With each cell division, like a photocopier trimming the margins of the original document, these protections lose a small fragment. This natural mechanism is one of the fundamental processes of biological aging.
The length of your telomeres could thus provide valuable information about your rate of aging and your biological age, which sometimes differs significantly from your chronological age. This measurement allows for evaluating the actual functional state of your organism beyond the simple counting of years.
Structure and function of telomeres
Telomeres correspond to tandem repeats of nucleotide sequences (TTAGGG) that decrease with each cell replication. These repeats contain no coding genes: their role is purely protective. By losing length division after division, they prevent essential genetic information from being altered or lost.
When telomeres reach a critical length, called the Hayflick limit, repair response pathways are activated to fix potential DNA damage. This activation leads to cell cycle arrest, which can result in senescence or programmed cell death. This safety mechanism prevents damaged cells from continuing to multiply and potentially becoming cancerous.
The link between telomeres and the aging process
Telomeres act like a biological clock governing the lifespan of cells, a concept known as the telomeric theory of aging. This theory establishes that the progressive shortening of telomeres is one of the central mechanisms of the aging process, even though it remains multifactorial.
When telomeres become too short to maintain their protective role, the cell stops dividing and functioning normally, becoming senescent. The accumulation of these senescent cells in tissues directly contributes to organismal aging, leading to a progressive deterioration of physiological functions.
Cellular senescence and organ aging
Senescent cells are cells that have permanently stopped their cell cycle under the influence of various stress signals. Contrary to what their name suggests, these cells do not age in the strict sense: they simply stop dividing while remaining metabolically active. With age, stem cells accumulate DNA damage and experience telomere shortening, which degrades the regenerative potential of tissues.
Most human cells die on average after 50 to 70 divisions, a limit known for decades as the “Hayflick limit.” This threshold explains why your tissues and organs gradually show signs of aging: the cells composing them reach their maximum renewal capacity.
Differences between men and women
A study showed that among 48-year-old men and women, there was a significant difference in telomere length of about 320 base pairs, equivalent to 8 to 10 biological years. Telomere shortening is faster in men than in women, which partly explains why life expectancy is on average higher in women. This difference suggests that women age biologically more slowly than men.
Scientists suggest that this difference is due to estrogens, which would promote telomere expression thanks to their anti-inflammatory and antioxidant activity. Female hormones thus play a protective role on cellular integrity and longevity.
Factors that accelerate telomere shortening
Beyond the natural process of cell division, several environmental and behavioral factors accelerate telomere shortening and precipitate biological aging. Identifying these factors allows concrete action to preserve your cellular capital.
The enemies of your telomeres
Certain lifestyle factors associated with an increased risk of developing cancer have also been linked to shortened telomeres: stress, smoking, physical inactivity, and a diet high in refined sugars. These habits create a hostile cellular environment that weakens the protective structures of your chromosomes.
Oxidative stress refers to assaults on cells by toxic particles called reactive oxygen species and free radicals, exacerbated by excess tobacco and alcohol, pollution, medication abuse, and pesticide exposure. People exposed to high oxidative stress and chronic inflammation have shorter telomeres than others.
Psychological stress is also linked to accelerated cellular aging, notably a decrease in telomerase activity and short telomeres. A study shows that women experiencing significant stress have telomere shortening corresponding to aging ten years more, demonstrating the considerable impact of chronic stress on your biological clock.
Diseases related to telomeric shortening
In humans, there are premature aging syndromes in which individuals have abnormally short telomeres due to mutations in genes coding for telomere maintenance proteins. These diseases, grouped under the name telomeropathies, demonstrate the direct link between telomere length and health.
People with telomeropathies exhibit early tissue regeneration deficiency, with bone marrow aplasia, skin pigmentation anomalies, premature gray hair or even alopecia, or fibrosis of certain organs such as lungs and liver. These pathologies illustrate the direct consequences of a telomeric dysfunction on the whole organism.
| Category | Negative factors | Observed consequences |
|---|---|---|
| Lifestyle | Smoking, sedentary behavior, lack of sleep, excessive alcohol consumption | Accelerated telomere shortening, increased risk of chronic diseases, premature aging |
| Diet | Excess refined sugars, processed meats, ultra-processed foods, sugary drinks, fried foods | Increased oxidative stress, chronic inflammation, reduced cellular repair capacity |
| Psychological factors | Chronic stress, persistent anxiety, social isolation, sleep disorders | Decreased telomerase activity, accelerated biological aging equivalent to several years |
| Environment | Air pollution, pesticide exposure, chemical toxins, excessive UV | DNA damage, increased free radicals, disruption of cellular repair mechanisms |
How to protect your telomeres?
The good news is that lifestyle changes, including a healthy diet, exercise, and stress reduction, have the potential to increase telomere length, reverse cellular aging, and reduce the risk of aging-related diseases. These natural interventions offer concrete empowerment over your longevity.
The diet that protects your telomeres
A diet rich in whole grains, seafood, legumes, vegetables, seaweed, and dairy products is positively associated with telomere length. This nutritional approach promotes cellular protection thanks to its supply of antioxidants and essential nutrients.
High consumption of legumes, nuts, seaweed, fruits, coffee, and dairy products is associated with longer telomeres, while high consumption of red meat, cold cuts, and sugary drinks is associated with shorter telomeres. Foods rich in antioxidants reduce the impact of oxidative stress and slow down the telomeric shortening process.
People living in Mediterranean countries have a longer and healthier life, with longer telomeres. The Mediterranean diet, rich in olive oil, fish, fresh fruits and vegetables, is an optimal dietary model to preserve your telomere capital.
Key nutrients for your telomeres include:
- Omega-3s: found in fatty fish, rapeseed oil, and walnut oil, they have protective anti-inflammatory properties
- Vitamins C and E: powerful antioxidants that neutralize free radicals and protect DNA
- B vitamins (B6, B9, B12): essential for cell replication and telomere maintenance
- Selenium and zinc: trace elements that support antioxidant defense mechanisms
- Polyphenols: found in green tea, turmeric, and berries, they fight inflammation
Physical activity: a major ally
The more people practiced physical activity during their leisure time, the longer their telomeres were. Regular training can activate telomerase in white blood cells and lengthen telomeres. Exercise acts as a natural stimulant of cellular protection mechanisms.
A study on men with prostate cancer showed that at least 30 minutes of walking six days a week, combined with a controlled diet and one hour daily of stress management, allowed telomeres to gain 10% of their length after five years. This research demonstrates the concrete potential of lifestyle interventions.
The benefits of physical activity on your telomeres are explained by several mechanisms: reduction of chronic inflammation, improvement of mitochondrial function, reduction of oxidative stress, and optimization of hormonal regulation. A balanced program combining cardiovascular exercises, muscle strengthening, and gentle practices like yoga offers the best results.
Stress management and sleep
Meditation with positive thinking can improve longevity and telomere length, with mood improvements associated with greater telomerase activity. Relaxation techniques, deep breathing, and mindfulness reduce stress and promote cellular health.
Sleep is also a crucial and often underestimated factor. Quality and sufficient quantity sleep enables your cells to regenerate effectively and maintains the hormonal balance necessary for telomere protection. Chronic sleep disturbances accelerate biological aging and compromise cellular repair mechanisms.
Telomerase: the longevity enzyme
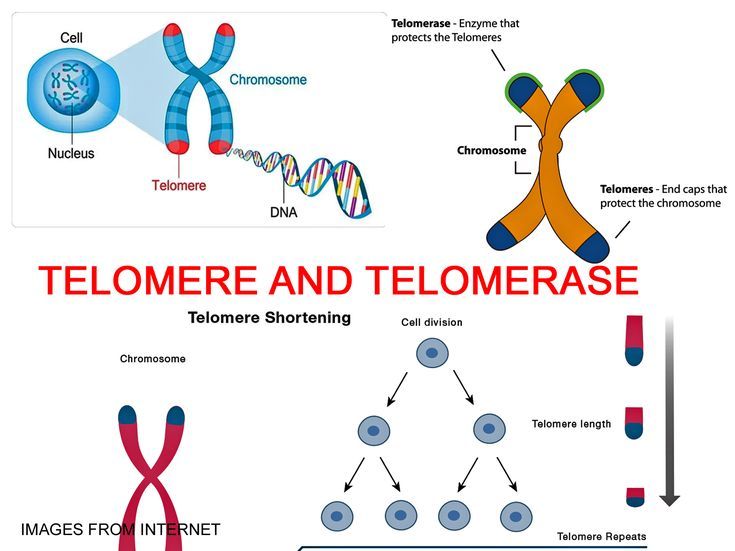
Telomerase is the enzyme naturally present in your body capable of maintaining and even lengthening your telomeres. This enzyme maintains telomere size division after division, offering cells that possess it an almost unlimited renewal capacity.
Functioning and localization of telomerase
Telomerase compensates for telomere shortening by synthesizing DNA that it adds to the telomere end. Cells expressing this protein are said to be immortal in the sense that they can proliferate without being limited to a finite number of cell divisions.
In humans, telomerase is active only in stem cells and those giving rise to sperm and egg cells. These stem cells ensure the continuous renewal of various tissues throughout life, such as skin, intestinal epithelium, or blood tissue. Without active telomerase, these tissues could not regenerate effectively.
Telomerase and longevity research
In October 2009, the Nobel Prize in Medicine was awarded to three American researchers, Elisabeth Blackburn, Carol Greider, and Jack Szostak, for the identification of telomerase. This major discovery opened new perspectives for understanding and potentially intervening in the aging processes.
Researchers at Harvard have shown that reactivating telomerase in genetically modified and prematurely aged mice led to a real rejuvenation of the animals with regeneration of damaged tissues. These promising results demonstrate the therapeutic potential of telomerase, even though its application in humans still requires extensive research.
The paradox of telomerase
Cancer cells exhibit particularly active telomerase, allowing an unlimited number of divisions associated with rapid and uncontrolled tumor growth. This paradox raises crucial questions: how to benefit from the protective effects of telomerase without increasing cancer risk?
An important question divides researchers: does taking a telomerase activator increase the risk of developing cancer? Current research is focused on understanding mechanisms that allow safe and targeted stimulation of telomerase, notably through natural approaches via diet and lifestyle rather than aggressive pharmacological interventions.
Measuring your telomeres: toward preventive medicine
Today, some laboratories offer telomere length measurements at prices ranging from 200 to 500 euros. In the future, these measurements could become common practice within the framework of preventive medicine for aging-related diseases.
Telomere length can be considered a “biological capital” constituted early in life, which plays an important role in the damage-repair balance at the cellular and tissue level, and can thus influence the aging trajectory. This measurement offers a view of your real biological age, potentially different from your chronological age.
Understanding and protecting your telomeres represents a concrete scientific approach to preserving your health and optimizing your longevity. By adopting an antioxidant-rich diet, engaging in regular physical activity, managing your stress, and limiting your exposure to toxins, you act directly on your cellular biological clock. These simple yet powerful habits constitute your best insurance for healthy aging and fully enjoying every stage of your life. The future of telomere research promises fascinating therapeutic advances, but today, you hold the keys to taking care of your cellular capital and building quality longevity.